Researchers at Oregon State University have shown that a chemical mechanism known as proton-hopping may provide a new method of energy storage for EVs and other high-power applications.
“Coming up with Faradaic electrodes that afford batteries energy density and capacitors power with excellent cycle life has been a big challenge. So far, most of the attention has been devoted to metal ions – starting with lithium and looking down the periodic table,” said lead researcher Xiulei (David) Ji.
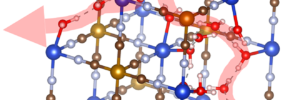
Instead, the researchers looked at proton-hopping, more formally known as the Grotthuss mechanism. It works when a an excess proton is introduced into a network of hydrogen bonds, such as water molecules. The proton will join the network and kick out a different proton. The newly displaced proton rejoins the network further down the line, and in turn kicks out a third proton, which kicks out a fourth, and so on. In this fashion, the proton moves all the way down the network.
“The motion is like a Newton’s cradle: correlated local displacements lead to the long-range transport of protons, which is very different from metal-ion conduction in liquid electrolytes, where solvated ions diffuse long distances individually in the vehicular manner,” said researcher Xianyong Wu.
The researchers conducted experiments to demonstrate that proton-hopping can create a highly efficient charging conduit, which could be useful for battery applications.
“If this mechanism is installed in battery electrodes, the proton doesn’t have to squeeze through narrow orifices in crystal structures,” Ji explained. “If we design materials with the purpose of facilitating this kind of conduction, this conduit is so ready – we have this magic proton highway built in as part of the lattice.”
Though promising, the research into proton-hopping batteries is still very preliminary.
“Without the proper technology involving research by materials scientists and electrical engineers, this is all purely theoretical,” Ji said. “Can you have a sub-second charge or discharge of a battery chemistry? We theoretically demonstrated it, but to realize it in consumer devices, it could be a very long engineering journey. Right now the battery community focuses on lithium, sodium, and other metal ions, but protons are probably the most intriguing charge carriers with vast unknown potentials to realize.”
Source: Oregon State University
Source: Electric Vehicles Magazine